And, for bonus points, how are they made?
These seem like an awfully important test piece. I’m pretty sure they’re just checking for glucose with some enzyme or something. But who knows, maybe its something simple or everyday? Are all brands using the same materials?
blood interacts with the chemicals on the test strip, the reaction creates a measurable electrical charge which changes depending on the glucose level in the blood. The meter reads the level of that electrical charge and converts it to a blood glucose reading in understandable numbers.
I believe most strips use glucose oxidase, an enzyme that produces gluconic acid from the glucose in the blood. But I haven’t researched new test strip tech in years.
I don’t think the strips themselves do anything but hold your blood for the actual tester you put them in.
I’m pretty sure the test strips strips probably do most of the testing, and the meter just reads the result.
All the test strips I’ve ever used hold the blood at the opposite end to the machine. The blood is intentionally kept away and on something that can easily be disposed of.
As far as I can tell, the bulk of the testing strip is a conductor to take a signal from the end with the blood to the tester, which makes it seem like there’s some sort of reaction on the blood end.
Considering they have an expiration date I don’t believe they’re inert.
Looking at the bottle my test strips are in, they contain: flavin adenine dinucleotide dependent dehydrogenase (from Aspergillus sp.); potassium ferricyanide; and other ingredients.
I’m just imagining the nutrition facts section for that bottle of test strips
If you’re talking about strips that look like this:

I believe that most brands use Benedict’s reagent: copper sulphate, sodium citrate, sodium carbonate. It interacts with reducing sugars (like glucose) and the colour goes from blue to light green to green to brown-green to brown-red, depending on concentration.
I’ve use quite a few brands, but I’ve never come across one that changed colors. Is that blue despite the blood sample?
These almost look more like ph or ketone test strips…
I think this might be a “yes, but no” kind of thing.
Yes, these are test strips. Yes, they change color to indicate a reading. Yes, they use chemical reactions to cause that color change.
AFAIK: No, these aren’t for testing blood. No, these don’t seem to be for consumption by an electronic meter. And no, I don’t think this is what OP was asking about.
Like, there’s probably some good info, but not for this thread specifically :P
Source: Pulling it straight out of my ass, but it is informed by my limited experience with medical test equipment, and much less limited experience with electronics.
Those are used with urine, not blood. Urine is yellow-ish, but since it’s just a drop, it doesn’t stain the strip; instead, the blue tip changes colour as it reacts with the glucose in the urine. Then you compare that coloured tip with a chart, and you get an idea of the concentration of glucose in your body. Picture related:
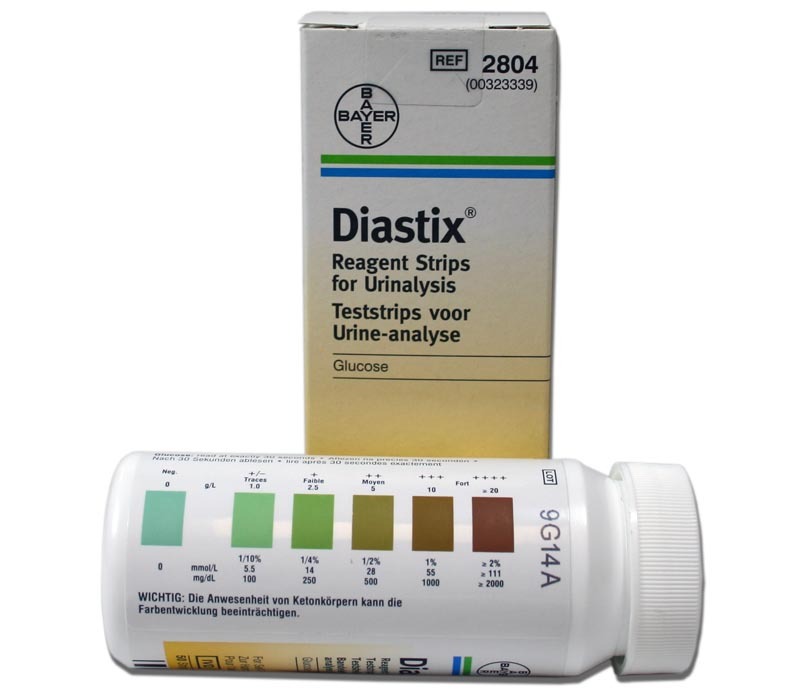
I remember seeing those bottles in my bathroom all the time as a kid (my sister is diabetic).
These almost look more like ph or ketone test strips…
It is roughly the same idea as the one behind the litmus test for pH, indeed. The difference is which substance you’re putting there (Benedict’s reagent vs. pH indicators) and which substance you’re testing for (glucose vs. H₃O⁺ / OH⁻).







